Investigating radioactivity
- We can use a Geiger counter to detect radioactivity. The counter clicks each time a particle of radiation from a radioactive substance enters the Geiger tube.
7.9 recall the sources of background radiation
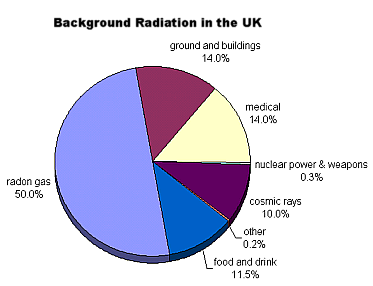
Radioactivity around us
When we use a Geiger counter, it clicks even without a radioactive source near it. This is due to background radiation from radioactive substances found naturally all around us.
Background radiation is:
- ionising radiation from space (cosmic rays)
- from devices e.g. X-ray tubes
- from radioactive substances in environment-some are present due to nuclear weapons testing and nuclear power stations, but most of it is from substances in the Earth (e.g. radon gas is radioactive and is a product of the decay of uranium in the ground)
Why are some substances radioactive?
Every atom has a nucleus made of protons and neutrons, and electrons orbit around in the space surrounding the nucleus.
Most atoms have a stable nucleus that doesn't change. But some are unstable, and they become stable by emitting alpha, beta or gamma radiation. An unstable nucleus is said to decay when it emits radiation. We can't tell when an unstable nucleus will decay. It is a random event that happens without anything being done to the nucleus.
 This means that they have a charge of +2, and a mass of 4
This means that they have a charge of +2, and a mass of 4
(the mass is measured in "atomic mass units", where each proton & neutron=1)
We can write them as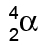 , or, because they're the same as a helium nucleus,
, or, because they're the same as a helium nucleus, 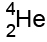 .
.
Alpha-decay occurs in very heavy elements, for example, Uranium and Radium.
These heavy elements have too many protons to be stable. They can become more stable by emitting an alpha particle.
Alpha particles have a large charge(+2), so they easily ionise other atoms that they pass. Ionising atoms requires energy, so alpha particles lose energy rapidly as they travel. Thus they have a range of only a few centimetres in air.
 Beta particles:
Beta particles:
Beta decay occurs in very "neutron-rich" elements, for example, Strontium-90 and Iodine-130. These elements are typically created in nuclear reactors.
These elements have too few protons and too many neutrons to be stable. They can thus become more stable by emitting a beta particle.
Beta particles have a charge of -1, and weigh only a tiny fraction of a neutron or proton. As a result,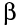 particles interact less readily with other atoms than alpha particles.
particles interact less readily with other atoms than alpha particles.
Thus beta particles cause less ionisation than alphas, and have a longer range, typically a few metres in air.
Gamma Rays
Gamma rays do not directly ionise other atoms, as they have no charge, although they may cause atoms to emit other particles which will then cause ionisation.
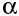 -particles and
-particles and 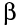 -particles pull electrons off atoms as they pass (we say they ionise the atoms), but
-particles pull electrons off atoms as they pass (we say they ionise the atoms), but 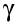 rays don't. This means that they do not lose much energy as they travel, as they do not interact as much with the matter they pass.
rays don't. This means that they do not lose much energy as they travel, as they do not interact as much with the matter they pass.
Therefore, gamma rays have a high penetrating power, and a very long range.
There is no such thing as a pure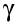 -ray source. Gamma rays are given off by most alpha emitters and beta emitters. If we want a source of pure gamma rays, we can get it by using a substance that emits both beta and gamma, and simply keep it in an aluminium container that stops the beta particles.
-ray source. Gamma rays are given off by most alpha emitters and beta emitters. If we want a source of pure gamma rays, we can get it by using a substance that emits both beta and gamma, and simply keep it in an aluminium container that stops the beta particles.
Useful gamma sources include Technetium-99, which is used as a "tracer" in medicine. This is a combined beta and gamma source, and is chosen because betas are less harmful to the patient than alphas (less ionisation) and because Technetium has a short half-life (just over 6 hours), so it decays away quickly and reduces the dose to the patient.
When an unstable nucleus decays, there are three ways that it can do so.
It may give out:-
It may give out:-
- an alpha particle (we use the symbol
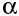 )
) - a beta particle (symbol
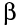 )
) - a gamma ray (symbol
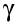 )
)
As far as we know, there are four fundamental forces in the universe.
In order of increasing strength, they are:-
- The Gravitational force
(very weak, only noticeable when large masses are involved) - The Electromagnetic force
(responsible for electrostatic attraction & repulsion,
also the behaviour of magnets) - The Weak Nuclear force
(actually pretty strong, but only operates over very short distances. Responsible for radioactive decay) - The Strong Nuclear force
(very strong, but very short range. Responsible for nuclear reactions such as fission).
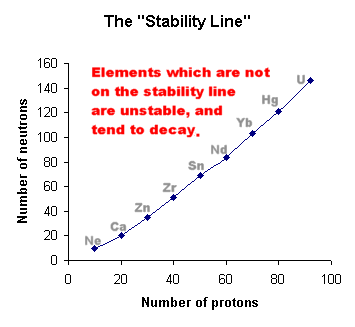
Inside any nucleus, the protons and neutrons are held together by the incredibly powerful "Strong Nuclear Force", which overcomes the electrostatic repulsion between the protons.
A balance exists between these two forces.
This means that certain numbers of protons and neutrons can make a stable nucleus, whilst other groupings will be more or less unstable. These will eventually decay to produce a more stable arrangement.
A balance exists between these two forces.
This means that certain numbers of protons and neutrons can make a stable nucleus, whilst other groupings will be more or less unstable. These will eventually decay to produce a more stable arrangement.

7.4 understand that alpha and beta particles and gamma rays are ionising radiations emitted from unstable nuclei in a random process
7.5 describe the nature of alpha and beta particles and gamma rays and recall that they may be distinguished in terms of penetrating power
Alpha particles
Alpha particles are made of 2 protons and 2 neutrons.
 This means that they have a charge of +2, and a mass of 4
This means that they have a charge of +2, and a mass of 4(the mass is measured in "atomic mass units", where each proton & neutron=1)
We can write them as
Alpha particles are relatively slow and heavy.
They have a low penetrating power - a sheet of paper will stop it.
Alpha particles ionise other atoms strongly as they have a large charge.
Alpha particles are made of 2 protons with 2 neutrons.
This means that when a nucleus emits an alpha particle, it loses 2 protons and so its atomic number decreases by 2.
Also, when a nucleus emits an alpha particle, its atomic mass decreases by 4 (2 protons + 2 neutrons)
These heavy elements have too many protons to be stable. They can become more stable by emitting an alpha particle.
Alpha particles have a large charge(+2), so they easily ionise other atoms that they pass. Ionising atoms requires energy, so alpha particles lose energy rapidly as they travel. Thus they have a range of only a few centimetres in air.
NOTE:
In alpha decay:
- atomic number decreases by 2
- atomic mass decreases by 4
 Beta particles:
Beta particles:
Beta particles have a charge of minus 1, and a mass of about 1/2000th of a proton. This means that beta particles are the same as an electron.
We can write them as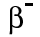 or, because they're the same as an electron,
or, because they're the same as an electron, 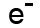 .
.
We can write them as
They are fast and light.
Beta particles have a medium penetrating power - they are stopped by a sheet of aluminium or plastics such as perspex.
Beta particles ionise atoms that they pass, but not as strongly as alpha particles do.
Under certain conditions, a neutron can decay, to produce a proton + an electron. The proton stays in the nucleus, whilst the electron is emitted at high speed.
This means that when a nucleus emits a 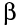 -particle:
-particle:
- the atomic mass is unchanged (neutrons and protons have same mass)
- the atomic number increases by 1 (addition of a proton)
This is because a neutron has changed into a proton (almost the same mass - we can ignore the tiny mass of the electron) and thus the number of protons has gone up.
Example: Strontium-90 undergoes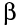 decay and forms Yttrium-90.
decay and forms Yttrium-90.
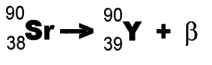

(This isn't the whole story - an almost massless particle called an "anti-neutrino" is also emitted. Furthermore, we are only considering "beta-minus" emission (negatively-charged electrons).
There is another type of beta decay, called "beta-plus", where a positively-charged electron (called a "positron") is emitted, along with a neutrino. But that's beyond IGCSE..)
These elements have too few protons and too many neutrons to be stable. They can thus become more stable by emitting a beta particle.
Beta particles have a charge of -1, and weigh only a tiny fraction of a neutron or proton. As a result,
Thus beta particles cause less ionisation than alphas, and have a longer range, typically a few metres in air.
Remember, in Beta decay :
- atomic number increases by one
- atomic mass unchanged.
Gamma Rays
Gamma rays have a high penetrating power - it takes a thick sheet of metal such as lead, or concrete to reduce them significantly.
Gamma rays do not directly ionise other atoms, as they have no charge, although they may cause atoms to emit other particles which will then cause ionisation.
We don't find pure gamma sources - gamma rays are emitted alongside alpha or beta particles. Strictly speaking, gamma emission isn't 'radioactive decay' because it doesn't change the state of the nucleus, it just carries away some energy.
Gamma rays (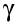 ) are electromagnetic waves, rather like X rays and radio waves.
) are electromagnetic waves, rather like X rays and radio waves.
(no mass and no charge.)
(no mass and no charge.)
After a nucleus has emitted an 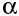 -particle or a
-particle or a 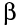 -particle, it may still have too much energy: we say it is in an "excited state".
-particle, it may still have too much energy: we say it is in an "excited state".
It can get rid of this energy by emitting a pulse of very high frequency electromagnetic radiation, called a gamma ray.
Therefore, gamma rays have a high penetrating power, and a very long range.
There is no such thing as a pure
Useful gamma sources include Technetium-99, which is used as a "tracer" in medicine. This is a combined beta and gamma source, and is chosen because betas are less harmful to the patient than alphas (less ionisation) and because Technetium has a short half-life (just over 6 hours), so it decays away quickly and reduces the dose to the patient.
Remember, in Gamma decay:-
- atomic number unchanged
- atomic mass unchanged.

:3 yesh I just finished revising radioactivity thanks to this XD
ReplyDeletehelps a lot
ReplyDeletethanks a lot!! its amazing
ReplyDeletethank you so much!! more informative than a IGCSE Physics book!!
ReplyDeletethank u so much sexy loved your notes
ReplyDeleteGreat stuff! The last part of Gamma rays was the one for me, it explained everything , from ionization to stuff that i wasn't curious enough to figure like Penetration .
ReplyDeletecan you please answer this question? "Describe how you would measure half-life of a radioactive isotope, assuming the half-life can be measured in minutes( rather than hours or years, for example). You should mention the apparatus you would use, the measurements that you would need to take and any safety precautions you must follow. (6 marks)
ReplyDelete<3 tenks a ton fr dese notes ya pretty creature!
ReplyDeletethank you
ReplyDeleteI sat with this stuff before my igcse exams and its a fab
ReplyDeletethanks a lot it us really helpful
ReplyDeletea good example of beta decay is when magnesium undergo beta decay, if it is a positron decay, the daughter nucleus is sodium-28, because positron decay does change the mass. if it a beta minus decay the daughter nucleus is aluminum-38.
ReplyDelete2020 still what I need.. Thanks Ms Michelle, helped me in the revision period of coronavirus
ReplyDeleteAwesome notes I AM YEAR 11 in 2021 and these notes are still helpful
ReplyDelete